TeleRPM BPM(Bluetooth® LE) 2022
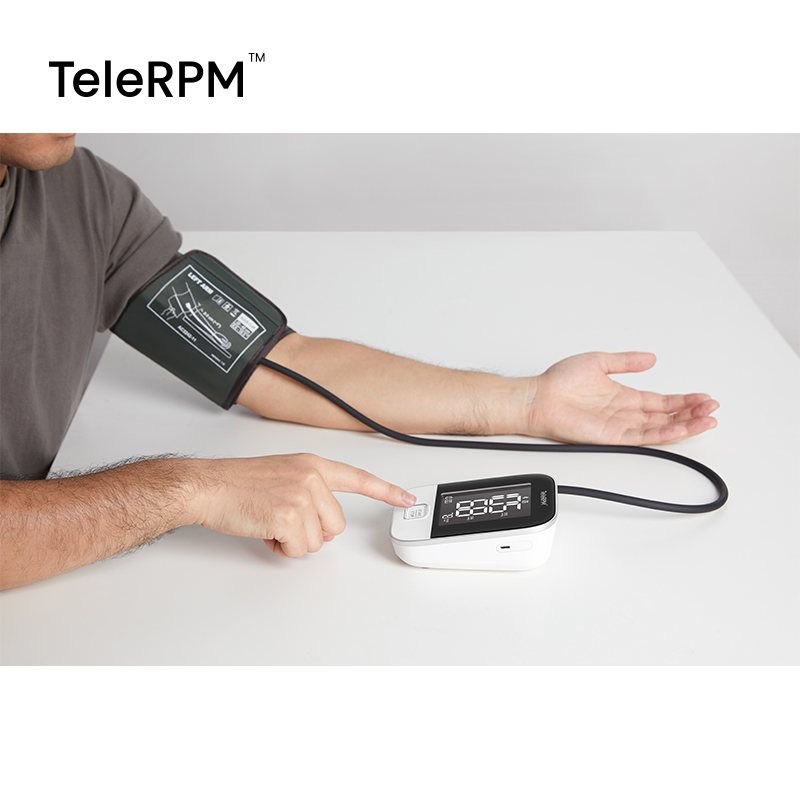
Bluetooth® 5.0 blood pressure monitor (Arm)
The Bluetooth® BP Monitor supports the latest Bluetooth® 5.0 connection with a lower consumption of energy and higher transmission speed, and applies to RPM health platform.
The Bluetooth® blood pressure monitor is extremely simple to use with one button and a replaceable Bluetooth® blood pressure cuff of various sizes.
TeleRPM blood pressure monitor not only measures your blood pressure accurately but also automatically transmits the data to your healthcare provider's platform, enabling remote monitoring and personalized care.